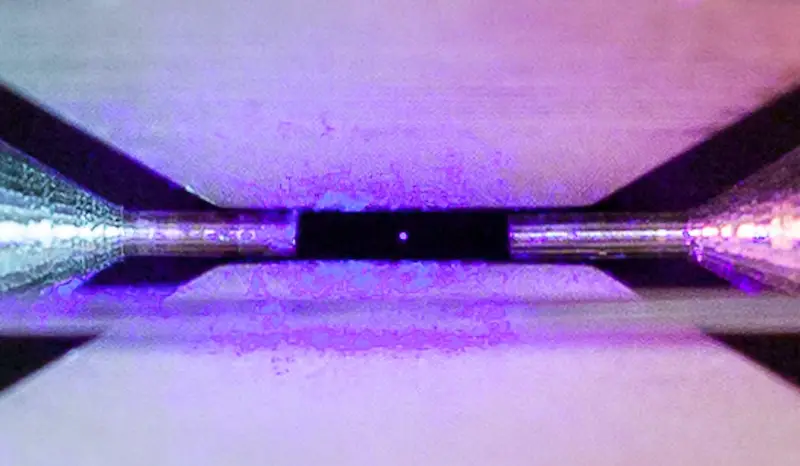
Have you ever wondered what the most basic building block of chemistry looks like? This captivating photo of an atom was captured by a David Nadlinger, a quantum physicist and PhD candidate at the University of Oxford. He titled his picture “Single Atom in an Ion Trap.”
The image earned David first prize in the Engineering and Physical Sciences Research Council (EPSRC) science photo contest.
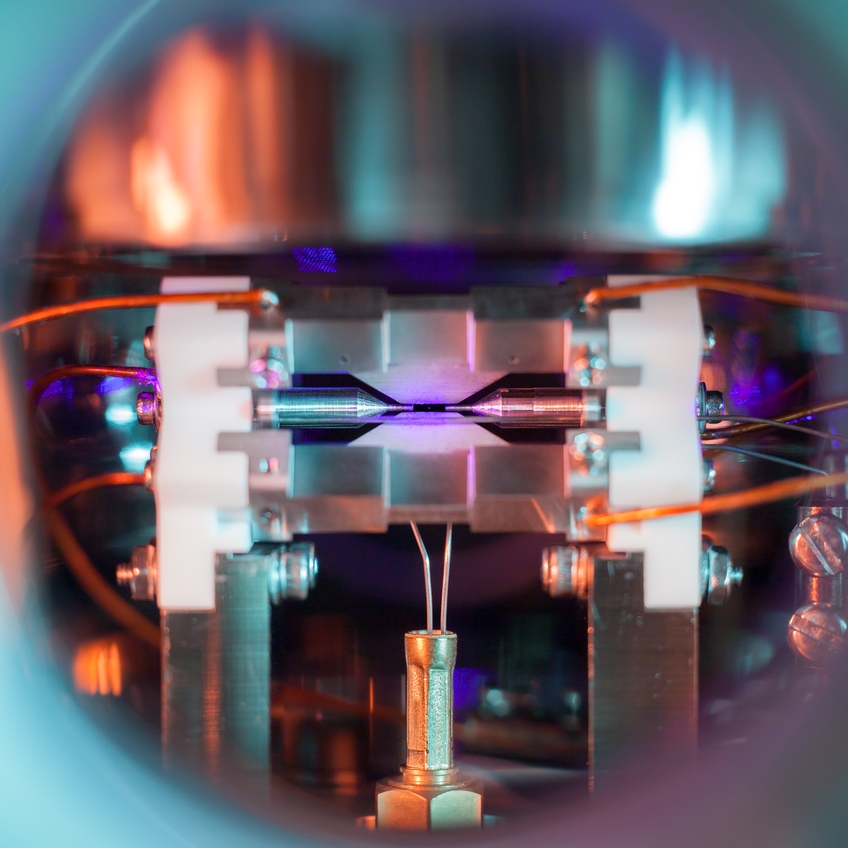
Perhaps the most amazing part about the photo is that David used a conventional camera to take the picture.
The EPSRC describes how this capture was able to occur:
When illuminated by a laser of the right blue-violet colour the atom absorbs and re-emits light particles sufficiently quickly for an ordinary camera to capture it in a long exposure photograph.
This particular atom comes from the strontium chemical element. Strontium is an alkaline earth metal and highly chemically reactive. It forms a dark oxide layer after contact with air.
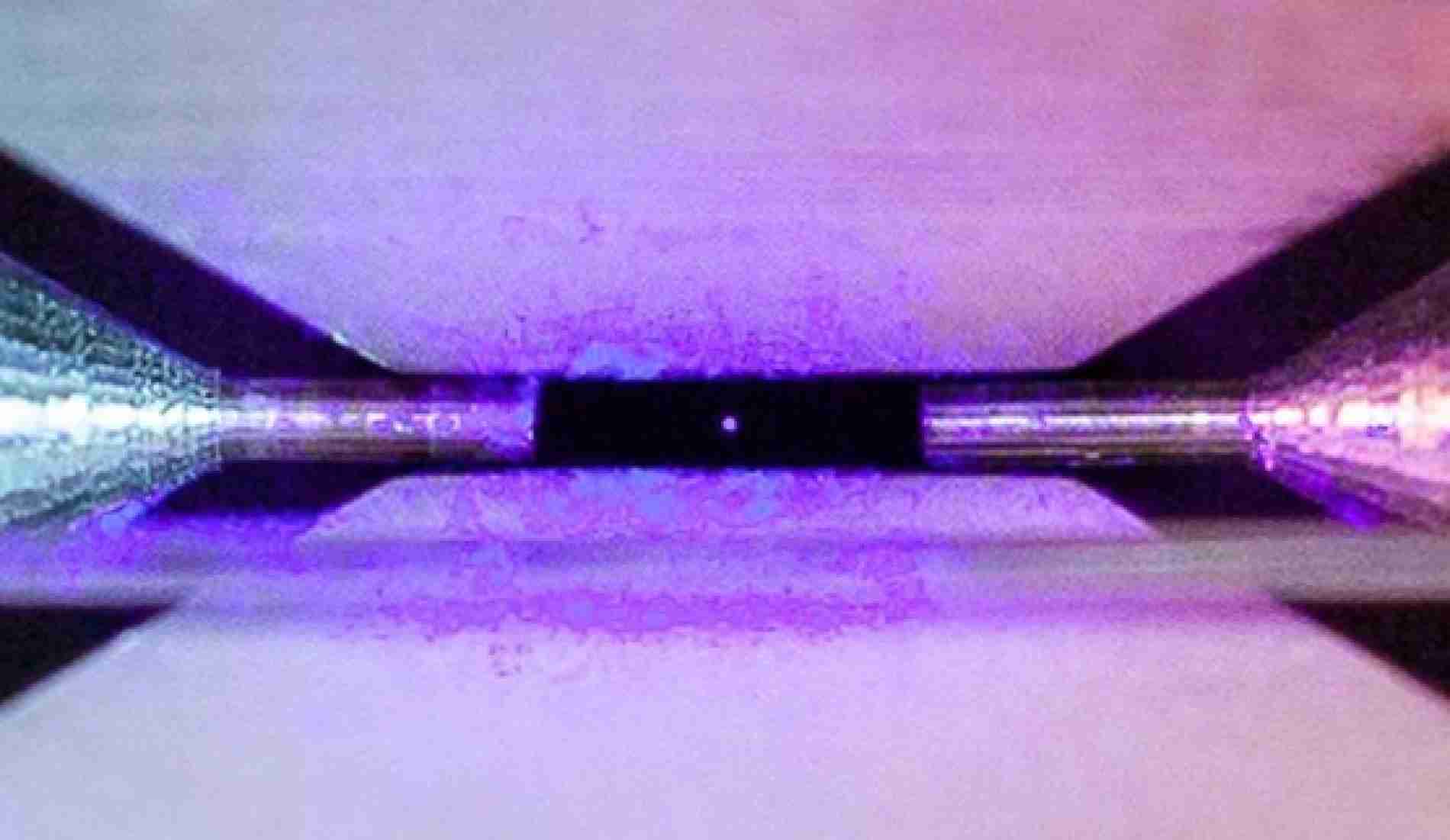
Below is the photo of an atom further zoomed in. The radiance producing the glowing effect is caused by a blue laser, while the particle floats motionless between two electrodes. The atom absorbs light fragments released by the laser and then re-emits them. This provides a nice view of the circular chemical building block.